new posts in all blogs
Viewing: Blog Posts Tagged with: periodic table, Most Recent at Top [Help]
Results 1 - 14 of 14
How to use this Page
You are viewing the most recent posts tagged with the words: periodic table in the JacketFlap blog reader. What is a tag? Think of a tag as a keyword or category label. Tags can both help you find posts on JacketFlap.com as well as provide an easy way for you to "remember" and classify posts for later recall. Try adding a tag yourself by clicking "Add a tag" below a post's header. Scroll down through the list of Recent Posts in the left column and click on a post title that sounds interesting. You can view all posts from a specific blog by clicking the Blog name in the right column, or you can click a 'More Posts from this Blog' link in any individual post.

By: JulieF,
on 8/21/2016
Blog:
OUPblog
(
Login to Add to MyJacketFlap)
JacketFlap tags:
#ACSPhiladelphia,
A Tale of Seven Scientists and a New Philosophy of Science,
atomic structure,
history of the periodic table,
John Dalton,
Books,
History,
chemistry,
periodic table,
*Featured,
Physics & Chemistry,
Science & Medicine,
eric scerri,
Dimitri Mendeleev,
American Chemical Society,
Add a tag
Our story has to begin somewhere and why not with the Manchester schoolteacher John Dalton who revived the atomic theory of the ancient Greek philosophers? In addition to supposing that the ultimate components of all matter were atoms, Dalton set about putting this idea on a quantitative foundation. He published the first list in which he compared the weights of the atoms of all the elements that were known at the time.
The post An egalitarian and organic history of the periodic table appeared first on OUPblog.

By: JulieF,
on 8/16/2016
Blog:
OUPblog
(
Login to Add to MyJacketFlap)
JacketFlap tags:
*Featured,
#ACSPhiladelphia,
bio-inorganic chemistry,
Oxford chemists,
R.J.P. Williams,
History,
chemistry,
British,
periodic table,
biochemistry,
Physics & Chemistry,
Science & Medicine,
Immanuel Kant,
evolutionary biology,
American Chemical Society,
periodic table of elements,
A World from Dust,
Ben McFarland,
How the Periodic Table Shaped Life,
Books,
Add a tag
Powell’s City of Books occupies 1.6 acres of retail floor space in downtown Portland, Oregon and is one of my favorite places in the world. My first time there, I searched out the chemistry shelves–and was slightly disappointed. I counted two cases of chemistry books sandwiched between biology and physics, which had eight cases each.
The post R.J.P. Williams and the advantages of thinking like a chemist appeared first on OUPblog.

By: JulieF,
on 3/15/2016
Blog:
OUPblog
(
Login to Add to MyJacketFlap)
JacketFlap tags:
periodic table of elements,
#ACSSanDiego,
A World from Dust,
Ben McFarland,
chemical rules,
CHON,
How the Periodic Table Shaped Life,
magnesium,
sulfur,
Books,
chemistry,
evolution,
Multimedia,
periodic table,
elements,
*Featured,
Physics & Chemistry,
Images & Slideshows,
Science & Medicine,
phosphorus,
Earth & Life Sciences,
chemical elements,
Add a tag
When people think of evolution, many reflect on the concept as an operation filled with endless random possibilities–a process that arrives at advantageous traits by chance. But is the course of evolution actually random? In A World from Dust: How the Periodic Table Shaped Life, Ben McFarland argues that an understanding of chemistry can both explain and predict the course of evolution.
The post How does chemistry shape evolution? appeared first on OUPblog.

By: Alice,
on 1/7/2016
Blog:
OUPblog
(
Login to Add to MyJacketFlap)
JacketFlap tags:
Books,
chemistry,
periodic table,
Editor's Picks,
*Featured,
Physics & Chemistry,
Science & Medicine,
eric scerri,
A Tale of Seven Elements,
chemical elements,
four super-heavy elements,
atomic numbers,
Charles Janet,
quarkonium matter,
Essays in the Philosophy of Chemistry,
Add a tag
The recent announcement of the official ratification of four super-heavy elements, with atomic numbers 113, 115, 117 and 118, has taken the world of science news by storm. It seems like there is an insatiable appetite for new information about the elements and the periodic table within the scientific world and among the general public.
The post Those four new elements appeared first on OUPblog.

By: JulieF,
on 8/17/2015
Blog:
OUPblog
(
Login to Add to MyJacketFlap)
JacketFlap tags:
Books,
History,
chemistry,
periodic table,
*Featured,
Physics & Chemistry,
Science & Medicine,
chemical elements,
American Chemical Society,
Marco Fontani,
Mariagrazia Costa,
Mary Virginia Orna,
The Lost Elements,
false discoveries,
lost elements,
scientific progress,
undiscovered elements,
Add a tag
How can an element be lost? Scientists, and the general public, have always thought of them as being found, or discovered. However, more elements have been “undiscovered” than discovered, more “lost” than found.
The post The undiscovered elements appeared first on OUPblog.

By: JulieF,
on 8/16/2015
Blog:
OUPblog
(
Login to Add to MyJacketFlap)
JacketFlap tags:
Books,
History,
chemistry,
periodic table,
*Featured,
Physics & Chemistry,
Science & Medicine,
eric scerri,
Dmitri Mendeleev,
A Tale of Seven Elements,
American Chemical Society,
G.N. Lewis,
Richard Abegg,
theory of valence,
valency,
Add a tag
One of the most interesting developments in the history of chemistry has been the way in which theories of valency have evolved over the years. We are rapidly approaching the centenary of G.N. Lewis’ 1916 article in which he proposed the simple idea that a covalent bond consists of a shared pair of electrons.
The post Who was Richard Abegg? appeared first on OUPblog.

By: Julie Fergus,
on 8/20/2014
Blog:
OUPblog
(
Login to Add to MyJacketFlap)
JacketFlap tags:
Books,
History,
This Day in History,
periodic table,
elements,
*Featured,
Physics & Chemistry,
history of science,
Science & Medicine,
Dmitri Mendeleev,
A Tale of Seven Elements,
chemical elements,
Emile Béguyer de Chancourtois,
John Reina Newlands,
Julius Lothar Meyer,
Add a tag
The discovery of the periodic system of the elements and the associated periodic table is generally attributed to the great Russian chemist Dmitri Mendeleev. Many authors have indulged in the game of debating just how much credit should be attributed to Mendeleev and how much to the other discoverers of this unifying theme of modern chemistry.
In fact the discovery of the periodic table represents one of a multitude of multiple discoveries which most accounts of science try to explain away. Multiple discovery is actually the rule rather than the exception and it is one of the many hints that point to the interconnected, almost organic nature of how science really develops. Many, including myself, have explored this theme by considering examples from the history of atomic physics and chemistry.
But today I am writing about a subaltern who discovered the periodic table well before Mendeleev and whose most significant contribution was published on 20 August 1864, or precisely 150 years ago. John Reina Newlands was an English chemist who never held a university position and yet went further than any of his contemporary professional chemists in discovering the all-important repeating pattern among the elements which he described in a number of articles.
Newlands came from Southwark, a suburb of London. After studying at the Royal College of chemistry he became the chief chemist at Royal Agricultural Society of Great Britain. In 1860 when the leading European chemists were attending the Karlsruhe conference to discuss such concepts as atoms, molecules and atomic weights, Newlands was busy volunteering to fight in the Italian revolutionary war under Garibaldi. This is explained by the fact that his mother was Italian descent, which also explains his having the middle name Reina. In any case he survived the fighting and set about thinking about the elements on his return to London to become a sugar chemist.
In 1863 Newlands published a list of elements which he arranged into 11 groups. The elements within each of his groups had analogous properties and displayed weights that differed by eight units or some factor of eight. But no table yet!
Nevertheless he even predicted the existence of a new element, which he believed should have an atomic weight of 163 and should fall between iridium and rhodium. Unfortunately for Newlands neither this element, or a few more he predicted, ever materialized but it does show that the prediction of elements from a system of elements is not something that only Mendeleev invented.
In the first of three articles of 1864 Newlands published his first periodic table, five years before Mendeleev incidentally. This arrangement benefited from the revised atomic weights that had been announced at the Karlsruhe conference he had missed and showed that many elements had weights differing by 16 units. But it only contained 12 elements ranging between lithium as the lightest and chlorine as the heaviest.
Then another article, on 20 August 1864, with a slightly expanded range of elements in which he dropped the use of atomic weights for the elements and replaced them with an ordinal number for each one. Historians and philosophers have amused themselves over the years by debating whether this represents an anticipation of the modern concept of atomic number, but that’s another story.
More importantly Newlands now suggested that he had a system, a repeating and periodic pattern of elements, or a periodic law. Another innovation was Newlands’ willingness to reverse pairs of elements if their atomic weights demanded this change as in the case of tellurium and iodine. Even though tellurium has a higher atomic weight than iodine it must be placed before iodine so that each element falls into the appropriate column according to chemical similarities.
The following year, Newlands had the opportunity to present his findings in a lecture to the London Chemical Society but the result was public ridicule. One member of the audience mockingly asked Newlands whether he had considered arranging the elements alphabetically since this might have produced an even better chemical grouping of the elements. The society declined to publish Newlands’ article although he was able to publish it in another journal.
In 1869 and 1870 two more prominent chemists who held university positions published more elaborate periodic systems. They were the German Julius Lothar Meyer and the Russian Dmitri Mendeleev. They essentially rediscovered what Newlands found and made some improvements. Mendeleev in particular made a point of denying Newlands’ priority claiming that Newlands had not regarded his discovery as representing a scientific law. These two chemists were awarded the lion’s share of the credit and Newlands was reduced to arguing for his priority for several years afterwards. In the end he did gain some recognition when the Davy award, or the equivalent of the Nobel Prize for chemistry at the time, and which had already been jointly awarded to Lothar Meyer and Mendeleev, was finally accorded to Newlands in 1887, twenty three years after his article of August 1864.
But there is a final word to be said on this subject. In 1862, two years before Newlands, a French geologist, Emile Béguyer de Chancourtois had already published a periodic system that he arranged in a three-dimensional fashion on the surface of a metal cylinder. He called this the “telluric screw,” from tellos — Greek for the Earth since he was a geologist and since he was classifying the elements of the earth.
Image: Chemistry by macaroni1945. CC BY 2.0 via Flickr.
The post The 150th anniversary of Newlands’ discovery of the periodic system appeared first on OUPblog.


By: Julie Fergus,
on 8/20/2014
Blog:
OUPblog
(
Login to Add to MyJacketFlap)
JacketFlap tags:
Books,
History,
chemistry,
This Day in History,
periodic table,
*Featured,
Physics & Chemistry,
Science & Medicine,
Dmitri Mendeleev,
chemical elements,
Marco Fontani,
Mariagrazia Costa,
Mary Virginia Orna,
The Lost Elements,
The Periodic Table's Shadow Side,
Add a tag
Dmitri Mendeleev believed he was a great scientist and indeed he was. He was not actually recognized as such until his periodic table achieved worldwide diffusion and began to appear in textbooks of general chemistry and in other major publications. When Mendeleev died in February 1907, the periodic table was established well enough to stand on its own and perpetuate his name for upcoming generations of chemists.
The man died, but the myth was born.
Mendeleev as a legendary figure grew with time, aided by his own well-organized promotion of his discovery. Well-versed in foreign languages and with a sort of overwhelming desire to escape his tsar-dominated homeland, he traveled the length and breadth of Europe, attending many conferences in England, Germany, Italy, and central Europe, his only luggage seemingly his periodic table.
Mendeleev had succeeded in creating a new tool that chemists could use as a springboard to new and fascinating discoveries in the fields of theoretical, mineral, and general chemistry. But every coin has two faces, even the periodic table. On the one hand, it lighted the path to the discovery of still missing elements; on the other, it led some unfortunate individuals to fall into the fatal error of announcing the discovery of false or spurious supposed new elements. Even Mendeleev, who considered himself the Newton of the chemical sciences, fell into this trap, announcing the discovery of imaginary elements that presently we know to have been mere self-deception or illusion.
It probably is not well-known that Mendeleev had predicted the existence of a large number of elements, actually more than ten. Their discoveries were sometimes the result of lucky guesses (like the famous cases of gallium, germanium, and scandium), and at other times they were erroneous. Historiography has kindly passed over the latter, forgetting about the long line of imaginary elements that Mendeleev had proposed, among which were two with atomic weights lower than that of hydrogen, newtonium (atomic weight = 0.17) and coronium (Atomic weight = 0.4). He also proposed the existence of six new elements between hydrogen and lithium, whose existence could not but be false.
Mendeleev represented a sort of tormented genius who believed in the universality of his creature and dreaded the possibility that it could be eclipsed by other discoveries. He did not live long enough to see the seed that he had planted become a mighty tree. He fought equally, with fierce indignation, the priority claims of others as well as the advent of new discoveries that appeared to menace his discovery.
In the end, his table was enduring enough to accommodate atomic number, isotopes, radioisotopes, the noble gases, the rare earth elements, the actinides, and the quantum mechanics that endowed it with a theoretical framework, allowing it to appear fresh and modern even after a scientific journey of 145 years.
Image: Nursery of new stars by NASA, Hui Yang University of Illinois. Public domain via Wikimedia Commons.
The post Dmitri Mendeleev’s lost elements appeared first on OUPblog.


By: Julie Fergus,
on 8/10/2014
Blog:
OUPblog
(
Login to Add to MyJacketFlap)
JacketFlap tags:
Books,
History,
giveaway,
periodic table,
bohr,
nicholson,
*Featured,
Physics & Chemistry,
John Nicholson,
Science & Medicine,
eric scerri,
periodic,
A Tale of Seven Elements,
Henry Moseley,
moseley,
American Chemical Society,
#ACSsanfran,
Anton den Broek,
Edmund Stoner,
Niels Bohr,
progress in chemistry,
Wolfgang Pauli,
electrons,
quantization,
broek,
Add a tag
By Eric Scerri
The past couple of years have seen the celebration of a number of key developments in the history of physics. In 1913 Niels Bohr, perhaps the second most famous physicist of the 20th century after Einstein, published is iconic theory of the atom. Its main ingredient, which has propelled it into the scientific hall of fame, was it’s incorporation of the notion of the quantum of energy. The now commonplace view that electrons are in shells around the nucleus is a direct outcome of the quantization of their energy.
Between 1913 and 1914 the little known English physicist, Henry Moseley, discovered that the use of increasing atomic weights was not the best way to order the elements in the chemist’s periodic table. Instead, Moseley proposed using a whole number sequence to denote a property that he called the atomic number of an element. This change had the effect of removing the few remaining anomalies in the way that the elements are arranged in this icon of science that is found on the walls of lecture halls and laboratories all over the world. In recent years the periodic table has even become a cultural icon to be appropriated by artists, designers and advertisers of every persuasion.
But another scientist who was publishing articles at about the same time as Bohr and Moseley has been almost completely forgotten by all but a few historians of physics. He is the English mathematical physicist John Nicholson, who was in fact the first to suggest that the momentum of electrons in an atom is quantized. Bohr openly acknowledges this point in all his early papers.
Nicholson hypothesized the existence of what he called proto-elements that he believed existed in inter-stellar space and which gave rise to our familiar terrestrial chemical elements. He gave them exotic names like nebulium and coronium and using this idea he was able to explain many unassigned lines in the spectra of the solar corona and the major stellar nebulas such as the famous Crab nebula in the constellation of Orion. He also succeeded in predicting some hitherto unknown lines in each of these astronomical bodies.
The really odd thing is that Nicholson was completely wrong, or at least that’s how his ideas are usually regarded. How it is that supposedly ‘wrong’ theories can produce such advances in science, even if only temporarily?
Science progresses as a unified whole, not stopping to care about which scientist is successful or not, while being only concerned with overall progress. The attribution of priority and scientific awards, from a global perspective, is a kind of charade which is intended to reward scientists for competing with each other. On this view no scientific development can be regarded as being right or wrong. I like to draw an analogy with the evolution of species or organisms. Developments that occur in living organisms can never be said to be right or wrong. Those that are advantageous to the species are perpetuated while those that are not simply die away. So it is with scientific developments. Nicholson’s belief in proto-elements may not have been productive but his notion of quantization in atoms was tremendously useful and the baton was passed on to Bohr and all the quantum physicists who came later.
Instead of viewing the development of science through the actions of individuals and scientific heroes, a more holistic view is better to discern the whole process — including the work of lesser-known intermediate figures, such as Nicholson. The Dutch economist Anton den Broek first made the proposal that elements should be characterized by an ordinal number before Moseley had even begun doing physics. This is not a disputed point since Moseley begins one of his key papers by stating that he began his research in order to verify the van den Broek hypothesis on atomic number.
Another intermediate figure in the history of physics was Edmund Stoner who took a decisive step forward in assigning quantum numbers to each of the electrons in an atom while as a graduate student at Cambridge. In all there are four such quantum numbers which are used to specify precisely how the electrons are arranged first in shells, then sub-shells and finally orbitals in any atom. Stoner was responsible for applying the third quantum number. It was after reading Stoner’s article that the much more famous Wolfgang Pauli was able to suggest a fourth quantum number which later acquired the name of electron spin to describe a further degree of freedom for every electron in an atom.
Eric Scerri is a full-time chemistry lecturer at UCLA. Eric Scerri is a leading philosopher of science specializing in the history and philosophy of the periodic table. He is also the founder and editor in chief of the international journal Foundations of Chemistry and has been a full-time lecturer at UCLA for the past fifteen years where he regularly teaches classes of 350 chemistry students as well as classes in history and philosophy of science. He is the author of A Tale of Seven Elements, The Periodic Table: Its Story and Its Significance, and The Periodic Table: A Very Short Introduction.
Chemistry Giveaway! In time for the 2014 American Chemical Society fall meeting and in honor of the publication of The Oxford Handbook of Food Fermentations, edited by Charles W. Bamforth and Robert E. Ward, Oxford University Press is running a paired giveaway with this new handbook and Charles Bamforth’s other must-read book, the third edition of Beer. The sweepstakes ends on Thursday, August 14th at 5:30 p.m. EST.
Subscribe to the OUPblog via email or RSS.
Subscribe to only physics and chemistry articles on the OUPblog via email or RSS.
The post Nicholson’s wrong theories and the advancement of chemistry appeared first on OUPblog.


By: Alice,
on 9/8/2013
Blog:
OUPblog
(
Login to Add to MyJacketFlap)
JacketFlap tags:
periodic table,
copenhagen,
elements,
*Featured,
Physics & Chemistry,
Science & Medicine,
eric scerri,
periodic,
scerri,
A Tale of Seven Elements,
atomic number,
chemical elements,
hafnium,
Henry Moseley,
Ida Noddack,
Lise Meitner,
Marguerite Perey,
X-ray radiation,
urbain,
moseley’s,
celtium,
moseley,
Add a tag
By Eric Scerri
After years of lagging behind physics and biology in the popularity stakes, the science of chemistry is staging a big come back, at least in one particular area. Information about the elements and the periodic table has mushroomed in popular culture. Children, movie stars, and countless others upload videos to YouTube of reciting and singing their way through lists of all the elements. Artists and advertisers have latched onto the iconic beauty of the periodic table with its elegant one hundred and eighteen rectangles containing one or two letters to denote each of the elements. T-shirts are constantly devised to spell out some snappy message using just the symbols for elements. If some words cannot quite be spelled out in this way designers just go ahead and invent new element symbols.
Moreover, the academic study of the periodic table has been undergoing a resurgence. In 2012 an International Conference, only the third one on this subject, was held in the historic city of Cuzco in Peru. Recent years have seen many new books and articles on the elements and the periodic table.
Exactly 100 years ago, in 1913, an English physicist, Henry Moseley discovered that the identity of each element was best captured by its atomic number or number of protons. Whereas the older approach had been to arrange the elements in order of increasing atomic weights, the use of Moseley’s atomic number revealed for the first time just how many elements were still missing from the old periodic table. It turned out to be precisely seven of them. Moseley’s discovery also provided a clear-cut method for identifying these missing elements through their spectra produced when any particular element is bombarded with X-ray radiation.
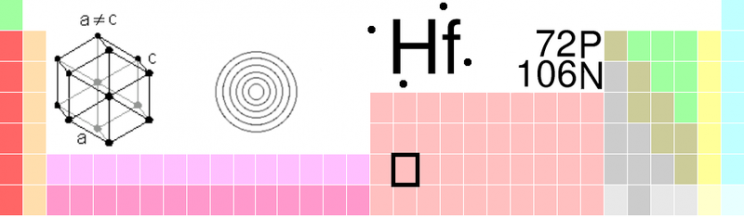
But even though the scientists knew which elements were missing and how to identify them, there were no shortage of priority disputes, claims, and counter-claims, some of which still persist to this day. In 1923 a Hungarian and a Dutchman working in the Niels Bohr Institute for Theoretical Physics discovered hafnium and named it after hafnia, the Latin name for the city of Copenhagen where the Institute is located. The real story, however, lies in the priority dispute that erupted initially between a French chemist Georges Urbain who claimed to have discovered this element, which he named celtium, as far back as 1911 and the team working in Copenhagen. With all the excesses of overt nationalism the British and French press supported the French claim because post-wartime sentiments persisted. The French press claimed, “Sa pue le boche” (It stinks of the Hun). The British press in slightly more restrained though no less chauvinistic terms announced that,
“We adhere to the original word celtium given to it by Urbain as a representative of the great French nation which was loyal to us throughout the war. We do not accept the name which was given it by the Danes who only pocketed the spoils of war.”
The irony was that Denmark had been neutral during the war but was presumably considered guilty by geographical proximity to Germany. Furthermore the French claim turned out to be spurious and the men from Copenhagen won the day and gained the right to name the new element after the city of its discovery.
Why are there so often priority debates in science? Generally speaking scientists have little to gain financially from their scientific discoveries. The one thing that is left to them is their ego and their claim to priority for which they will fight to the last. Another possibility is that women first discovered three or possibly four of the seven elements left to be discovered between the old boundaries of the periodic table (when it was still thought that there were just 92 elements). The three who definitely did discover elements were Lise Meitner, Ida Noddack, and Marguerite Perey from Austria, Germany, and France respectively. This is one of several areas in science where women have excelled, others being observational astronomy, research in radioactivity, and X-ray crystallography to name just a few.
One hundred years after the race began, these human stories spanning the two world wars continue to fascinate and provide new insight in the history of science.
Eric Scerri is a leading philosopher of science specializing in the history and philosophy of the periodic table. He is also the founder and editor in chief of the international journal Foundations of Chemistry and has been a full-time lecturer at UCLA for the past fourteen years where he regularly teaches classes of 350 chemistry students as well as classes in history and philosophy of science. He is the author of A Tale of Seven Elements, The Periodic Table: A Very Short Introduction, and The Periodic Table: Its Story and Its Significance. Read his previous blog posts.
Subscribe to the OUPblog via email or RSS.
Subscribe to only physics and chemistry articles on the OUPblog via email or RSS.
Image credit: Image by GreatPatton, released under terms of the GNU FDL in July 2003, via Wikimedia Commons.
The post Understanding the history of chemical elements appeared first on OUPblog.


By: Alice,
on 3/6/2012
Blog:
OUPblog
(
Login to Add to MyJacketFlap)
JacketFlap tags:
elements,
*Featured,
Physics & Chemistry,
higher education,
Science & Medicine,
this day in world history,
periodic,
atomic weight,
Dmitri Mendeleev,
John Alexander Reina Newlands,
Lothar Meyer,
Russian Chemical Society,
mendeleev,
mendeleev’s,
dmitri,
periodicity,
chemist,
style”,
History,
chemistry,
This Day in History,
periodic table,
Add a tag
This Day in World History
March 6, 1869
Mendeleev’s Periodic Table presented in public

Russian chemist Dmitri Mendeleev. Source: NYPL.
On March 6, 1869,
Dmitri Mendeleev’s breakthrough discovery was presented to the
Russian Chemical Society. The chemist had determined that the known elements — 70 at the time — could be arranged by their atomic weights into a table that revealed that their physical properties followed regular patterns. He had invented the periodic table of elements.
In his early twenties, Mendeleev had intuited that the elements followed some kind of order, and he spent thirteen years trying to discover it. In developing his system, he drew on the data and ideas of scientists around the world. Two — Lothar Meyer and British chemist John Alexander Reina Newlands — had published ideas about the periodicity of elements. But Mendeleev’s addressed every known element, which theirs had not.
His system also surpassed the others because he accounted for gaps in the sequence of elements. Mendeleev said that an element would be discovered to fill each gap and even predicted the properties of those elements. The discovery of the one of these missing elements — gallium, in 1875 — helped spur wide acceptance of Mendeleev’s system.
Later work showed that Mendeleev’s reliance on atomic weight to determine periodicity is not completely correct. While atomic weight tends to increase as one moves from element to element, there are exceptions. Mendeleev also did not have the theoretical understanding to explain why the elements exhibited these periodic characteristics. Nevertheless, his achievement marked an important milestone in the understanding of the physical world.
Mendeleev did not personally present his breakthrough to the Chemical Society. Ill on the day of the meeting, he asked a colleague to deliver the report.
Interestingly, the date celebrated for this event reflects Russia’s use of the “Old Style” Julian calendar. According to the “New Style” Gregorian calendar — not adopted in Russia until after 1918 — Mendeleev’s periodic table was presented twelve days later, on March 18.
“This Day in World History” is brought to you by USA Higher Education.
You can subscribe to these posts via RSS or receive them by email.



By: Nicola,
on 11/30/2011
Blog:
OUPblog
(
Login to Add to MyJacketFlap)
JacketFlap tags:
A Very Short Introduction,
*Featured,
Physics & Chemistry,
Environmental & Life Sciences,
Darmstadt,
Science & Medicine,
technetium,
Cn,
copernicium,
darmstadtium,
eric scerri,
International Union for Pure and Applied Physics,
IUPAP,
Nicholas Copernicus,
Rg,
roentgenium,
Wilhelm Konrad Roentgenm,
periodic,
scerri,
VSI,
periodic table,
Ds,
Add a tag
By Eric Scerri
As far back as I can remember I have always liked sorting and classifying things. As a boy I was an avid stamp collector. I would sort my stamps into countries, particular sets, then arrange them in order of increasing monetary value shown on the face of the stamp. I would go to great lengths to select the best possible copy of any stamp that I had several versions of. It’s not altogether surprising that I have therefore ended up doing research and writing books on what is perhaps the finest example of a scientific system of classification – the periodic table of the elements. Following degrees in chemistry I wrote a PhD thesis in the history and philosophy of science and specialised in the question of whether chemistry has been explained by quantum mechanics. A large part of this work dealt with the periodic table, the explanation of which is considered as one of the major triumphs of quantum theory, and the notion of atomic orbitals.
As I often mention in public lectures, it is curious that the great 20th century physicist, Ernest Rutherford, looked down on chemistry and compared it to stamp collecting. But we chemists had the last laugh since Rutherford was awarded the Nobel Prize for chemistry and not for his beloved field of physics.
In 2007 I published a book called The Periodic Table, Its Story and Its Significance, which people tell me has become the definitive book on the subject. More recently I was asked to write a Very Short Introduction to the subject, which I have now completed. Although I first thought this would be a relatively easy matter it turned out not to be. I had to rethink almost everything contained in the earlier book, respond to comments from reviewers and had to deal with some new areas which I had not developed fully enough in the earlier book. One of these areas is the exploration of elements beyond uranium or element number 92, all of which are of a synthetic nature.
At the same time there has been a veritable explosion of interest in the elements and the periodic table especially in the popular imagination. There have been i-Pad applications, YouTube videos, two highly successful popular books, people singing Tom Leher’s element song in various settings as well as artists and advertisers helping themselves to the elegance and beauty of the periodic table. On the scientific side, elements continue to be discovered or more precisely synthesised and there are official deliberations concerning how the recently discovered elements should be named.
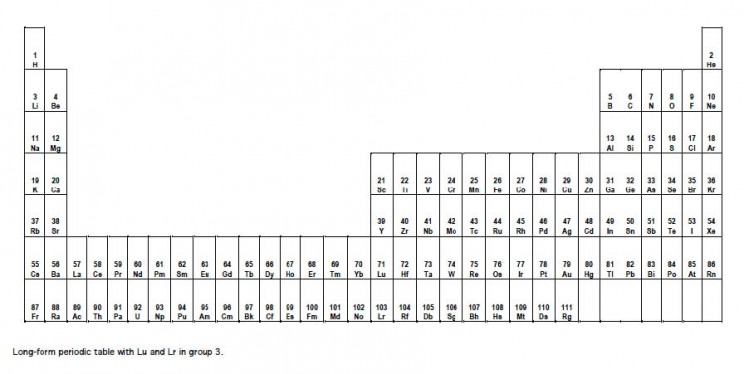
On November 4th The International Union for Pure and Applied Physics (IUPAP) officially announced that elements 110, 111 and 112 are to be known officially as darmstadtium (Ds), roentgenium (Rg) and copernicium (Cn). The names come from the German city of Darmstadt where several new elements have been artificially created; Wilhelm Konrad Roentgenm, the discoverer of X-rays; and the astronomer Nicholas Copernicus who was one of the first to propose the heliocentric model of the solar system. Of the three names it is the last one that has caused the most controversy. Apart from honouring a great scientist it was chosen because the structure of the atom broadly speaking resembles that of a miniature solar system in which the nucleus plays the role of the sun and the electrons behave as the planets do, an idea that originated with the work of Rutherford incidentally. Except
I wonder how many times students have been directed to complete a table for homework or on an exam. Chemists have an incomplete table that they are trying to complete:
the Periodic Table of the Elements, or the Periodic Table, for short. Uranium, element number 92 is the last naturally occurring element in the Periodic Table. Elements beyond number 92, called the transuranium elements, have all been produced in laboratories. The first transuranium element, neptunium (#93) was produced in 1940 at the University of California, Berkeley, by Edwin McMillan and Phillip Abelson by exposing uranium oxide to neutrons from a
cyclotron. The last one,
copernicium (#112) was officially recognized in 2009.
On June 6, 2011, the Joint Working Party on the Discovery of Elements of the International Union of Pure and Applied Chemistry (
IUPAC) and the International Union of Pure and Applied Physics (
IUPAP) announced the addition of
two new elements to the Periodic Table—element 114 and element 116. For now, element 114 is called ununquadium and element 116 is called ununhexium. These names are based on their atomic numbers. By officially acknowledging the collaboration between researchers from Lawrence-Livermore National Laboratory in California and Russia’s Joint Institute for Nuclear Research in Dubna, these researchers will get to suggest names for the new elements. The names will go through a review process before being adopted and the elements will be assigned a symbol by the IUPAC Council.
Scientists produced these elements by bombarding curium (#96) atoms with calcium (#20) nuclei. In a few milliseconds, element 116 decays into element 114 which lasts about half a second before decaying into copernicium (#112). In other experiments element 114 was produced by bombarding plutonium (#94) with calcium nuclei. Notice that 96 + 20 = 116 and 94 + 20 = 114.
There are three more elements waiting to be recognized: 113, 115, and 118. According to IUPAC, “Review of the claims associated with elements 113, 115, and 118 are at this time not conclusive and evidences have not met the criteria for discovery.” As soon as I hear anything more, I will let you know.

By:
Aline Pereira,
on 11/15/2007
Blog:
PaperTigers
(
Login to Add to MyJacketFlap)
JacketFlap tags:
bullying,
Peace,
Picture Books,
Books at Bedtime,
Eventful World,
Glin Dibley,
Allen Shamblin,
Anti-bullying week,
Don-t Laugh at Me,
Operation Respect,
reading to children,
Peter Yarrow,
Raeding Zone,
Steve Seskin,
Steve Seskin,
Add a tag
 Next week is Anti-Bullying Week in the UK, when school-children throughout the country will take part in activities to help them:
Next week is Anti-Bullying Week in the UK, when school-children throughout the country will take part in activities to help them:
“grow up with their respect of self and others intact, be fine participant citizens and, perhaps most importantly, become peacemakers in their hearts.”
This quotation comes from Peter Yarrow’s afterword of a remarkable picture book of Steve Seskin and Allen Shamblin’s deeply incisive but simple song Don’t Laugh at Me. The words of the song have become increasingly familiar since first being written just over ten years ago: but set here with Glin Dibley’s hauntingly expressive illustrations, and with certain words in the text highlighted in red, even young children will be able to respond to it, using their innate sense of justice to pull out the essence of the song’s message.
Be prepared for taking your time over it: each line triggers all sorts of questions and discussion. Reading this book to your own children or to a class of young children is a beautiful way to introduce them to the notion that “difference” should make no difference. They will appreciate the juxtapositions in the illustrations, like the one of the boy in a helmet in a wheelchair – in that order: the wheelchair is actually the last thing you notice.
There’s also a cd at the back and kids of all ages will enjoy listening to the song, performed so gently and meditatively by the song-writers themselves.
Peter Yarrow, quoted above, founded Operation Respect and a percentage of the sales of the book goes to their “Don’t Laugh at Me” project… And there’s also a Spanish edition. Reading Zone has just placed it in in its Top Ten Picture Books. So what are you waiting for?












We gain two new elements and lose a planet. Don't these scientists have better things to do? At least it is interesting to know what they are up to.