By Eric Scerri
After years of lagging behind physics and biology in the popularity stakes, the science of chemistry is staging a big come back, at least in one particular area. Information about the elements and the periodic table has mushroomed in popular culture. Children, movie stars, and countless others upload videos to YouTube of reciting and singing their way through lists of all the elements. Artists and advertisers have latched onto the iconic beauty of the periodic table with its elegant one hundred and eighteen rectangles containing one or two letters to denote each of the elements. T-shirts are constantly devised to spell out some snappy message using just the symbols for elements. If some words cannot quite be spelled out in this way designers just go ahead and invent new element symbols.
Moreover, the academic study of the periodic table has been undergoing a resurgence. In 2012 an International Conference, only the third one on this subject, was held in the historic city of Cuzco in Peru. Recent years have seen many new books and articles on the elements and the periodic table.
Exactly 100 years ago, in 1913, an English physicist, Henry Moseley discovered that the identity of each element was best captured by its atomic number or number of protons. Whereas the older approach had been to arrange the elements in order of increasing atomic weights, the use of Moseley’s atomic number revealed for the first time just how many elements were still missing from the old periodic table. It turned out to be precisely seven of them. Moseley’s discovery also provided a clear-cut method for identifying these missing elements through their spectra produced when any particular element is bombarded with X-ray radiation.
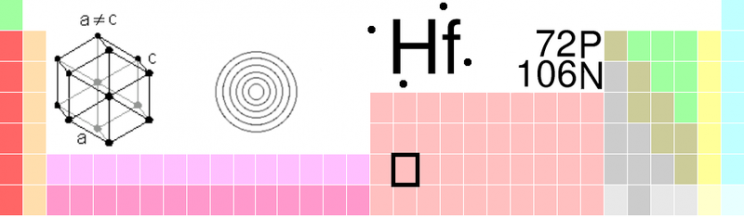
But even though the scientists knew which elements were missing and how to identify them, there were no shortage of priority disputes, claims, and counter-claims, some of which still persist to this day. In 1923 a Hungarian and a Dutchman working in the Niels Bohr Institute for Theoretical Physics discovered hafnium and named it after hafnia, the Latin name for the city of Copenhagen where the Institute is located. The real story, however, lies in the priority dispute that erupted initially between a French chemist Georges Urbain who claimed to have discovered this element, which he named celtium, as far back as 1911 and the team working in Copenhagen. With all the excesses of overt nationalism the British and French press supported the French claim because post-wartime sentiments persisted. The French press claimed, “Sa pue le boche” (It stinks of the Hun). The British press in slightly more restrained though no less chauvinistic terms announced that,
“We adhere to the original word celtium given to it by Urbain as a representative of the great French nation which was loyal to us throughout the war. We do not accept the name which was given it by the Danes who only pocketed the spoils of war.”
The irony was that Denmark had been neutral during the war but was presumably considered guilty by geographical proximity to Germany. Furthermore the French claim turned out to be spurious and the men from Copenhagen won the day and gained the right to name the new element after the city of its discovery.
Why are there so often priority debates in science? Generally speaking scientists have little to gain financially from their scientific discoveries. The one thing that is left to them is their ego and their claim to priority for which they will fight to the last. Another possibility is that women first discovered three or possibly four of the seven elements left to be discovered between the old boundaries of the periodic table (when it was still thought that there were just 92 elements). The three who definitely did discover elements were Lise Meitner, Ida Noddack, and Marguerite Perey from Austria, Germany, and France respectively. This is one of several areas in science where women have excelled, others being observational astronomy, research in radioactivity, and X-ray crystallography to name just a few.
One hundred years after the race began, these human stories spanning the two world wars continue to fascinate and provide new insight in the history of science.
Eric Scerri is a leading philosopher of science specializing in the history and philosophy of the periodic table. He is also the founder and editor in chief of the international journal Foundations of Chemistry and has been a full-time lecturer at UCLA for the past fourteen years where he regularly teaches classes of 350 chemistry students as well as classes in history and philosophy of science. He is the author of A Tale of Seven Elements, The Periodic Table: A Very Short Introduction, and The Periodic Table: Its Story and Its Significance. Read his previous blog posts.
Subscribe to the OUPblog via email or RSS.
Subscribe to only physics and chemistry articles on the OUPblog via email or RSS.
Image credit: Image by GreatPatton, released under terms of the GNU FDL in July 2003, via Wikimedia Commons.
The post Understanding the history of chemical elements appeared first on OUPblog.